Future Hangs in the Balance as Healthcare Industries Pass Tipping Points
By Brian Hudock, Partner
July 2013
www.tompkinsinc.com
The pharmaceutical & healthcare industries have reached their biggest crossroads.
An ever-evolving, global marketplace in healthcare industries has created a challenging business landscape with multiple paths from which to choose. At this critical crossroads sit healthcare and pharmaceutical, waiting for the right diagnosis and a guide to move forward.
This combined, complex industry includes healthcare, pharmaceutical, and bio-technology, as well as the medical device and medical product sectors. How these industries identify themselves and navigate the crossroads-individually and jointly-will determine their success in the domestic and international marketplace as single sector leaders or as multi-market healthcare service providers in the coming decade.
As you read the daily headlines, it is clear all healthcare industries are challenged by a growing online sales presence, increasing consumer pressures, uncertain global dynamics, market shifts, and profit erosion factors. In fact, one could say these companies are at a series of crossroads with no single right path for all, but instead, multiple options based upon organizational strategic direction.
The traditional business questions that were once heavily debated no longer matter at these new supply chain crossroads. The traditional distribution network models and fulfillment operations are now obsolete.
New strategy questions begin to appear, such as how to get the right medications and medical products to a growing and aging population. This strategy is especially complex as the population now lives in a global environment that is highly regulated and poses substantial marketplace obstacles. Moreover, drugs are becoming more specialized and consumers now have multiple means of technology that allow them to access information 24 hours a day with the new “Amazon expectation” of same- or next- day delivery.
Therefore, the essential question with supply chain structure becomes: what supply chain configuration is needed to match an all-encompassing new global business strategy, local market challenges, and changing consumer demands? At this crossroads, business strategy must come before structure, or the path forward leads to failure.
SIGNALS & CHALLENGES
Consider the pharmaceutical industry. It has evolved from what was many years ago a self-contained, complete supply chain to the current multi-partner, multichannel system that involves contract suppliers, contract manufacturers (formulations through fill and pack), internal production, international partners, global transportation, country-specific distribution via third-party partners, internal operations, and even joint partners. Add to this complex structure the demand-driven need for products to be shipped directly to specific customers, along with the additional flow exclusively through wholesale and re-packaging channels. This is further complicated by country-specific pricing, drug approvals/acceptance, patent enforcement, distribution, and tax rules, to name a few.
What can pharmaceutical and healthcare related companies do to ensure success and avoid costly strategy and supply chain mistakes that will negatively impact long-term market sales and brand reputation?
At this crossroads, there are clear directional signs to guide your team for the near term and beyond. Most signs revolve around security and regulatory guidelines, such as global track and trace compliance for finished goods. Additional road signs are in the works to mark the path all the way back to active pharmaceutical ingredients (API) manufacturing and supply chain partners. Once the final rules are in place, anyone not addressing global track and trace compliance standards will struggle to succeed elsewhere.
Some crossroads with less well-marked choices may appear daunting, such as the struggle to define future markets for viable sourcing opportunities, obtaining profitable business volumes and margins, and tackling intellectual property and patent protections.

Understanding the crossroads challenges of pharmaceutical and other healthcare companies in the medical product and medical device markets has been a focus of Tompkins International for many years. Tompkins has developed a different approach to strategic planning to not only make the right directional choices at each crossroad, but to also build dynamic, responsive, and highly efficient supply chains for all regions of the globe.
This paper discusses industry pressures and tipping points, as well as how to best address them at this crucial time. The goal is to help you develop an internal roadmap to get ahead of and attack issues, rather than react to them on a daily basis.
INDUSTRY EVOLUTION
The pharmaceutical and healthcare industries have become significantly more complex over the past few years. Most experts project that the pace of change will likely continue to accelerate as countries struggle with healthcare costs, governments attempt to determine the best and most cost-effective treatments, and emerging markets reach consumer maturity, as well as become significant marketplace competitors.
The regulatory aspect of the marketplace may implode drug distribution rules as the traditional “Western” model (manufacturer to wholesaler/distributor to pharmacy/clinic) may morph into something new (manufacturer to consumer-direct or manufacturer to e- pharmacy to consumer-direct) in all markets.
Even in North America, what is to stop an online retailer similar to Amazon or Walmart (who has announced they want to be the number one health provider), from becoming the biggest global online pharmacy in a few short years? These retail giants have the financial resources necessary to make such a bold move, and buying a small script fulfillment player to obtain licensing could happen tomorrow.
As drugs and devices become more customized and the aging U.S. population demands that their prescriptions/supplies are delivered weekly versus monthly in individualized daily packaging, who is better to support this? Is it the current players, or top tier online retailers like Amazon or Walmart in partnership with a well-established script fulfillment organization? Who is best positioned for next- or same-day delivery?
The major online retailers’ ability and capability to supply next-day delivery and/or highly efficient regularly scheduled automatic consumer replenishment is already part of their business models. They have the systems, expertise, facilities, financial means, and desire to increase revenues and volume. The only advantages these organizations currently lack are licenses and regulatory expertise. They already understand consumer wants, needs, and demands.
You may be shaking your head in disbelief, or perhaps are suddenly feeling ill as you contemplate this huge challenge to turn your operations upside down. Everything you know today will change quickly if a single large online retailers or other “new age” provider storms into the distribution and fulfillment channel that involves the pharmaceutical and healthcare industry.
All those well-proven supplier and partner agreements that are in place will no longer matter; you will be forced to renegotiate volumes with much more aggressive players. If you do not participate, your competitor’s product will be featured and pushed as alternatives.
Just imagine: “This Week’s Special Deal, two for one Cholesterol X”. This offer may seem far-fetched, but with many emerging markets moving rapidly into the e-marketplace, some version of this type of fulfillment is very likely to become a reality in the near future.
Secondly, with the continued shift to more bio-tech and customized drugs, what value does shipping specialized drugs via secondary channels offer? Many of these are administered by clinicians and physicians or by a patient home care provider. Do current direct distribution rules make sense in this healthcare cost reduction environment? Are you prepared to distribute directly to your customer internally or through your third-party logistics partners handling distribution?

As drugs and devices become more customized and the aging U.S. population demands that their prescriptions/supplies are delivered weekly versus monthly in individualized daily packaging, who is better to support this?
Even while watching the marketplace closely, we cannot say for certain which of these scenarios will come to pass in the next few months or years, but all signs indicate that a substantial change is looming. This change will be amplified by increasing government regulation, price controls, and market interference, which will further force companies to evaluate, plan for, and monitor changes more closely in healthcare and pharmaceutical related industries.
INDUSTRY PRESSURES, SUPPLY CHAIN RESPONSES
The industry is quite simply at the apex of the “perfect storm” in terms of managing profitability and market share. Consider the recent and pending patent expirations on numerous blockbusters, the increased completive capabilities of the generic players, and growing regulatory hurdles (slower approvals and recent failures).
The concept of lean-based continuous improvement-traditionally favored in other industries to reduce supply chain costs and improve efficiency-is gaining traction across pharmaceutical and healthcare supply chains to improve margins. Tompkins International has been providing a lean continuous improvement focus for more than three decades. We understand that continuous improvement in healthcare industries must include supply chain control and cost reduction efficiency improvements in order to succeed.
For those of us who work in the healthcare industry and focus on supply chain issues, we have seen these pressures evolve across less regulated healthcare sectors and in other industries in multiple ways and at differing rates of adaptation. We have learned from other industries, such as retail and consumer products, that a highly adaptable, agile, and cost-effective supply chain operation can be created by:
-
Employing successful continuous improvement and lean initiatives
-
Understanding the end goal of identified requirements
-
Anticipating the most probable and potential supply chain issues
Of course, companies cannot build supply chains to only handle the immediate pressures. Much like playing the cards on the table in poker before they are all turned up, you will generally end up with a losing hand once everything is dealt out, no matter how much you bet upfront.
Pharmaceutical and healthcare companies have always worked to be efficient and to provide unmatched service levels, but service risk factors historically outweighed lean efforts that only focused on cost savings. Currently, instead of just focusing on full compliance with regulations, maintaining “safe inventory levels”, and getting product securely to market, cost savings are an equal focus. Product handling and control factors still outweigh simple cost reduction, but the justification formulas and open budgets are much tighter.
This philosophical change in thinking has not reduced or impacted the focus on better compliance and security initiatives involving product and patient safety concerns or full regulatory compliance. Companies must continue to be diligent and focus on protecting the integrity of their products and maintain well- designed security initiatives including track and trace, serialization, anti- counterfeiting efforts, and other security initiatives incorporated into their supply chains to mitigate risk.
Even one negative incident can adversely affect patient trust, brand image, and margins due to intense media scrutiny, regulatory fines/shutdowns, and lawsuits.
Security and process control compliance are necessary not only for distribution, but also for overall supply chains in the areas of sourcing, transportation, quality, manufacturing, packaging, distribution, and returns. As pharmaceutical and healthcare-related companies begin to diversify markets and focus on new and specific segments, the reconfiguration of supply chain infrastructure and systems must be quick, complete, compliant, and fully secure.
NINE KEY TIPPING POINTS
What has led pharmaceutical & healthcare to its current crossroads?
We have touched on a few tipping points above, but let’s take a look at the list as a whole.
-
Government Intervention: Controlling rising healthcare costs to balance budgets through the implementation of price controls/price setting, drug evaluation and selection, negotiated discounts, and selective patent enforcement are just a few methods being employed. At what profit margin or percentage of product line does it makes sense to enter or stay in the market, including having operations in any particular country?
-
The Highly Informed Consumer: As the availability of information for self-diagnostics and the free flow of information across social networks expands up-to-the minute product and treatment options, the ability to market directly to the “patient” is rapidly growing. This new way of engaging and interacting with consumers about this type of product completely alters the old model of physician-based education. However, working to keep the information accurate and in front of potential users has become a 24/365 effort.
-
Globalization: Demand for specific regional remedies to address country-specific illnesses across the globe (developed and emerging markets) must be balanced across research focus, market size, and humanitarian benefits. It is not only about profits, but also sales volumes which can drive research investment long-term. In addition, specialization of drugs must be carefully weighed against the market’s ability to support the costs to develop and produce them.

As the availability of information for self- diagnostics and the free flow of information across social networks expands up- to- the minute product and treatment options, the ability to market directly to the “patient” is rapidly growing.
-
Evolving Business Model: The role of wholesalers, traditional distribution, the impact of generics, and the role of the Internet and online retailing are all changing product marketing, sales channels, and the overall supply chain model. No single model fits all aspects of the new model, and it is projected that what we know now is unrelated to what the future may hold. Adaptive, flexible, and efficient models must be conceived for tomorrow, and this needs to start today.
-
Mergers and Acquisitions: There will be fewer larger companies globally vying for more consumers, but at the same time, they will be competing with government controls, specialized markets, and shorter product life cycles. The smaller companies will continue to be aggressively pursued and integrated into larger organizations, including pharmaceutical, bio-tech, medical device, medical products, generics, and other healthcare products, to create the balanced model for global markets.
-
Non-traditional Pharmaceutical Distribution (including Online): The number of potential product distribution channels from clinical trials to consumer-direct fulfillment is exponential and will likely vary across all markets and business segments. The ability to leverage all resources will become a predominant supply chain skill set.
-
Integrated Healthcare Providers: What is the right mix of healthcare products to build both consistent and low cost supply chains, to offer customers a compelling menu to drive sales, and provide volume leverage in countries? This will drive the constant change in supply chains for many organizations. Just because you are spinning off something today does not necessarily mean you are not acquiring new product lines tomorrow.
-
Supply Chain Security: Global security initiatives should not be viewed solely from a regulatory perspective, but also from a brand integrity and patient safety perspective. The human risks involved with the healthcare industry-particularly pharmaceuticals and medical products-raise the bar in visibility, performance, and operating margins.
-
Internal versus External Operations: Evaluating the option of leveraging logistics service providers (LSPs) to focus on core strengths and reduce internal costs is an industry best practice. However, the risks and trade-offs must be fully understood before outsourcing any particular business function.
FOUR PILLARS OF HEALTHCARE INDUSTRY SUCCESS
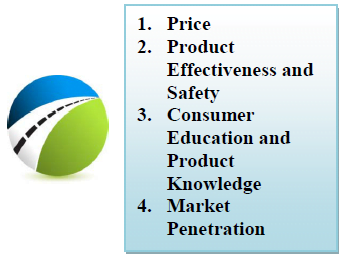
The pharmaceutical and healthcare industries are focused on improving the lives of people, offering treatments, cures, devices, and remedies aimed at making daily life better. However, the business of healthcare and all industry segments’ future success is based on the following factors:
-
Price: Government healthcare limits, pre-negotiated rates, insurance approvals, and completive products will impact prescription choices greatly in all markets. Determining what a specific market will accept as a fair price will change annually as contracts are renegotiated. Consumers will become much more focused on treatment choices as their insurance coverage becomes more limited and their out-of pocket costs rise. Picking the “low cost” choice (within physician prescribed options) will be further complicated by increased e-com/mail order and replenishment options versus in-store discounts and same-day convenience.
-
Product Effectiveness and Safety: Research and product development will still be the key to long-term success via internal research and development or acquisition and partnerships. However, having complete control and visibility of ever-extending supply chains and being compliant with global track and trace initiatives will be equally critical to maximize the peak sales window for formulations. Any disruptions will open the door to competitors’ products, accelerated patent expirations, or exclusivity losses, as well as consumer distrust.
-
Consumer Education and Product Knowledge: Education of physicians was once enough to sell the benefits of a drug, but with the open Internet and increased social media discussions, self diagnoses are commonplace today. Having a complete and accurate product description is not only necessary, but a best practice requirement. Monitoring results and feedback constantly to identify inaccuracies, real product issues/side effects, and other potential benefits are also a necessity. The ability to connect with patients and better educate them on choices about their treatment must be a combination of online and provider-based decisions.
-
Market Penetration: Perhaps the most difficult issue to identify upfront is how to enter a market, become the product of choice, and maintain a profitable business model. With government intervention, unique market dynamics, and product supply chain challenges, not all markets are viable. Understanding the market challenges, risks, and patient population is critical. A few of the most important marketplace factors are:
-
Asia: The Chinese market growth across the board has dipped below double digits and will likely not accelerate anytime soon. But huge investments in manufacturing capacity and development continue in anticipation of the world’s largest customer base reaching maturity. Complex business rules and the changing Chinese government’s interference in the free market do not appear to be stabilizing anytime soon. This needs to be settled before it severely affects foreign investments.
-
Europe: The marketplace in Europe is currently driven by government price controls and often favors internal country players. The challenge is that each country has taken a slightly different path. How to best leverage margin versus volume on sales has become a much more complex equation dependent upon very sophisticated sales and operation planning tools. In addition, internal supply chains in Europe are driven by logistics service partners as much as by sourcing partners.
-
North America: The Affordable Care Act, or government mandated healthcare for all, is now a reality. What this means for price controls, Medicare reimbursements, drug approvals, and healthcare consumption remains to be seen. But 40 to 65 million more eligible citizens in the highest margin pharmaceutical marketplace will impact demand, drive volume to generics and lower cost options when available.
-
Other Emerging Global Markets: Limited supply chain partners and shifting government policies continue to make other Latin American and African markets risky and difficult to penetrate on any type of large scale, so they remain costly to operate and offer low margin marketplaces.
-
LESSONS LEARNED
-
Do Strategy First: The first step to reaching any new position of performance is to develop the right strategy for your business. There is no substitute or shortcut to this step. Where many healthcare producers fail is by not defining their strategy well, or not being able to communicate it to all stakeholders- investors, management, employees, and customers. Basically, strategy has three components:
-
The Target Market: Who are our targeted customers? How are they segmented? What do they need and expect?
-
The Products, Services, and Value Proposition: What do we provide, and why should our target customers buy these from us? How do we distinguish our value proposition? How do we know how we are doing?
-
The Capabilities: What do we need to deliver? How should we do it and is there a compelling theme that differentiates us? What are our critical success factors?
Examples of obsolete strategies include:
-
Blockbuster exclusive drugs that dominate the market
-
High prices set by manufacturers (not the market/payers)
-
One-size-fits-all devices and products
-
Rapid drug approval and patent protection in all markets
-
Across the board coverage for all products
-
Doctor-driven treatment decision control
-
-
Have the Right Model: Which is better: To provide a single product line backed by a focused organization, or a broad portfolio maximizing the healthcare market needs and potentially minimizing market segment uncertainty? In the recent past, both strategies and multiple variants of each were seen as some companies divided to improve performance and profitably by division. Others have continued to diversify and look for ways to maximize their footprint across the healthcare marketplace.
In addition, knowing when to enter, exit, and selectively service given markets will drive many other business decisions, including investments in infrastructure, partnerships, and procurement.

The first step to reaching any new position of performance is to develop the right strategy for your business.
-
Look Forward, Not Backward: What has been the norm in the past or worked in developed markets has almost no relation to emerging markets and cost conscious developed markets. Looking forward means developing multi-partner supply chains that can adapt quickly and react to market surges and contractions (e.g., new product launches on a regular basis). Adaptability, along with efficiency improvements and a cost reduction focus, will drive future success and failures.
-
Build World-Class Supply Chains: Having the most effective or best priced product in the market only matters if you can deliver safely to healthcare providers and patients when they need it. Customizing the product flow, tracking to local markets, and leveraging internal and external capabilities are the building blocks for success. Supply chain leadership will be responsible for continuing to redefine these objectives and adapt them to the global market.
-
Achieve Supply Chain Excellence: Fulfilling consumer needs through multichannel solutions for all markets is a key to overall supply chain excellence. This includes having the right inventory policies, balancing global production and infrastructure, maintaining reliable and multiple sourcing partners, and leveraging external partners and channels for fulfillment. The only manner in which to do this is through partnership and information exchange, thus maintaining security and ensuring that product is available to the market without building massive inventories of raw materials, work-in-process or finished goods.
FIVE STEPS FOR INTEGRATED GLOBAL OPERATIONS EXCELLENCE
A growing number of global pharmaceutical and healthcare markets are entered via mergers and acquisitions. Global integration is often challenging when merging existing entities that have standalone systems, non-standardized processes, and different ideas about customer service than the acquiring parent organization.
Many acquisitions and partnerships are entered into to obtain and leverage existing market knowledge and presence, with the belief that these local firms actually know how to service the market more effectively.
However, local firms may not meet the standards, controls, or brand protection required for long-term success. In order to create a truly integrated global operation, there are five key steps to follow:
-
Strategy: Going in without a plan in any new marketplace or healthcare business category is like walking through a live minefield and hoping you leave with all your limbs. It is essential to understand the marketplace, the operational capabilities of the business you are acquiring or partnering, the local government, the reality of the local infrastructures, and the capabilities of the local management team. There is no substitute for a thorough due diligence. In fact, it is the only way to develop a strategy to improve, integrate, and leverage local strengths into core organization standards.
-
Fulfillment Plan: Understanding the local market distribution supply chain will drive the fulfillment plan design to optimize sales channel management. Most markets will be multichannel with a mix of retail pharmacy direct, clinic direct, wholesaler/3PL distribution, and even e-commerce. The fulfillment plan must be capable of supporting all local channels within regulatory rules and do so efficiently. This will be driven by how efficiently the distribution and fulfillment systems are configured.
-
Processes and Organization: Implementing best practices and processes begins with a strong organization and leadership team that supports the global culture and values, as well as builds and trains the local team. If any country or regional operation establishes independent processes, then the global integration strategy will fail. This is not to say that each country will not have unique requirements and should not maintain regional personalities to best serve the local markets, but they should all support the global standards and leverage corporate tools.
-
Technologies: One of the key steps to global integration is to establish and use common information technology systems and technologies (e.g., ERP platforms, warehouse management systems, product and location labeling, quarantine procedures). The mandate to be able to track and trace product from suppliers to customers does not accommodate piecemeal data collection or product identification. In the case of systems, there must be standardized processes internally and with any external partners.
-
Lean Continuous Improvement: With the ever-changing market dynamics, government pressures, acquisitions, and divestitures, the only thing that is certain is change. Many organizations use this as an excuse for not implementing improvements because they are too focused on managing “change.” This is the wrong approach. Every change and new requirement should be an opportunity and driver for continuous improvement in all operations-from warehouse to manufacturing to distribution. Each change must look at building additional flexibility into the process, leveraging better infrastructures, and improving the operations team’s skills both tactically and strategically.
Tompkins has worked for more than 35 years across many industries and learned that when these five steps are followed, not only do organizations produce significant improvements, but they also build a culture of continuous improvement. Each success drives the local and global teams forward, eager to discover the next improvement opportunity.
NINE KEY SUCCESS FACTORS
In order to measure how well your organization is reacting to each of the crossroads as you reach and navigate them, compare your progress against the following success factors:
-
Market Intelligence: Do you know what you customers want and where the business will go next? Monitoring social media, close coordination with your customers and partners, and government involvement are critical. If you are waiting on change or you lack market intelligence, you will experience supply chain problems.
-
Customer Centric: Are you servicing your customers by providing the right product in the right form at the right time? Are you fully protecting the supply chain integrity, and is customer safety and convenience a priority? Are you balancing market needs and product benefits fairly against profit margins across the globe?
-
Global Player/Emerging Markets: Being a global player means serving the global market. Exiting the market due to price limitations must be rigorously evaluated, but your competition will fill the gap if you choose to leave. Adapting your market strategy may be the better solution (but not always).
-
Product Confidence: Does the market trust your brand and the promises you are making? Supply chain integrity and consistent global practices will help build this confidence. A failure in any market will impact product confidence globally, so developing into a global integrated operation is critical.
-
Real-Time Visibility: Part of the product and market confidence will be driven by high service levels and product traceability. Having standardized product labeling, systems, and supplier and downstream partner connectivity will provide the visibility to all aspects of the supply chains and enable rapid reactions to any supply chain disruption around the globe.
-
Demand-Driven: Pharmaceuticals must be readily available in order to be effective and provide patient/customer benefits. However, the days of building piles of inventory to minimize risk are gone as drug values have risen and profit margins have decreased. Moving to a demand-driven organization is critical to reducing inventory (raw materials and finished goods) carrying costs, expiration, and general facility holding costs.
-
Supply Chain Execution: All road signs point back to supply chains and the impact of market and operational gaps. However, the benefits of fully integrated supply chains are only as effective as the supply chain team itself. Successful local and global teams and tools must be managed by a centralized team of planners, managers, and logisticians who refine it daily. Change is constant, and each day holds a new challenge

Being a global player means serving the global market. Exiting the market due to price limitations must be rigorously evaluated, but your competition will fill the gap if you choose to leave.
-
Technology: Information must be shared in real time on a common platform, and tools need to be in place to identify and react to problems in any area around the globe. Having the technology to support country-to-country business will be system and technology driven. Technology should further facilitate communication and visibility.
-
Flexibility: This factor for success is perhaps the most difficult for supply chain planners to grasp and deal with consistently. Understanding how to build in flexibility for change is key to developing not only a best practice driven operation, but in wisely investing capital into infrastructure that is adaptable quickly and cost effectively. Understanding the purpose and limitations of equipment and systems will also be a driving success factor.
MOVE BEYOND THE CROSSROADS

Developing a true multichannel, integrated global organization across multiple healthcare industry segments – or even a single market segment such as pharmaceuticals – has developed into a daunting task for supply chain and business planners. However, organizations across multiple industries have been successfully building the infrastructure and tools for many years.
The healthcare and pharmaceutical industries are not so different from other industries at this crossroads, but the risks and oversight are greater. There is significantly more government intervention and regulation of markets, and product traceability and security have a much stronger focus. Moreover, multichannel, country-by-country product distribution is a new factor that is becoming more important for the industry to navigate at this crossroads.
It is also important to understand and address who will be your upstream and downstream partners, which market channel and new channel partners will fulfill customer orders, and what they will demand from you. It is essential to build the infrastructure and technologies required to service markets, reduce costs, and know where products have been and are going. This not only requires internal capabilities, but also experienced external expertise from partners and supply chain experts.
There is no doubt that all healthcare- related companies, from pharmaceutical to daily use medical products, now live in exciting times. But these are not easy times.
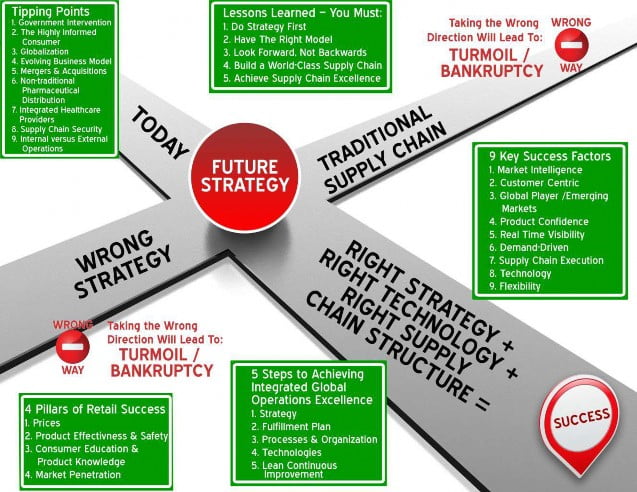
To further unravel opportunities and discover solutions, see the graphic presented on the next page. This illustration reveals the true nature of the crossroads and the path to achieving success in healthcare and pharmaceutical industries.
About Tompkins International
Tompkins International transforms supply chains to help create value for all organizations. For more than 35 years, Tompkins has provided end-to-end solutions on a global scale, helping clients align business and supply chain strategies through operations planning, design and implementation. The company delivers leading-edge business and supply chain solutions by optimizing the Mega Processes of PLAN- BUY-MAKE-MOVE-STORE-SELL. Tompkins supports clients in achieving profitable growth in all areas of global supply chain and market growth strategy, organization, operations, process improvement, technology implementation, material handling integration, and benchmarking and best practices. Headquartered in Raleigh, NC, USA, Tompkins has offices throughout North America and in Europe and Asia. For more information, visit www.tompkinsinc.com.
This paper is the result of Tompkins International’s research of public information. There is no information presented that comes from any proprietary source. Tompkins International does not discuss information about their clients unless that information has been published.


